EPA Places Ban on Minor Greenhouse Gas

Carbon dioxide and methane may grab all the headlines, but several other human-produced gases absorb energy in the infrared, too. The IPCC keeps a running list of radiatively relevant “species” that contains many lesser known agents active in our atmosphere. (See Wikipedia’s page for the most current breakdown.) Criteria for making it on the list include the molecule’s radiative efficiency (i.e. its ability to trap heat energy), its overall concentration in the atmosphere, and its residence time or atmospheric life. Suffice to say, the shorter this list, the better for the planet’s thermostat.
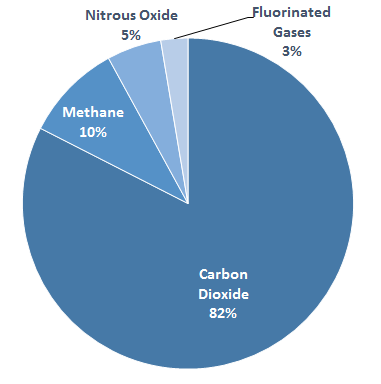
As part of the Climate Action Plan unveiled in 2013, the Obama administration have been working closely with the EPA on curbing greenhouse pollution. In addition to proposing new standards on carbon and setting ambitious renewables targets, the EPA has also targeted a family of greenhouse gases known as fluorinated gases, or F-gases. This suite of synthetic substances — which encompasses hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6) and nitrogen trifluoride (NF3) — is widely used in refrigeration and HVAC systems, and in the manufacture of semiconductors. Through their various commercial and industrial applications, enough of it wends its way into the atmosphere to account for 3 percent of US greenhouse emissions.
This month the EPA finalized new rules regulating the use of high-impact HFCs. The ban targets HFCs for two reasons. Chiefly, they are the most common F-gas in use today and, second, their forcing potential is several thousand times that of CO2 on a molecule-for-molecule basis. Left unchecked, HFC emissions are slated to nearly triple by 2030.
But, one might ask, if HFCs are such a burden on the climate, why were they ever approved in the first place? To answer this question, we need to go back about thirty-five years and look at their predecessor, CFCs.
In the 1980s it became clear that a class of refrigerants called CFCs (short for chlorofluorocarbons) was involved in eroding the ozone layer above the poles, allowing greater quantities of UV radiation to leak down into the lower atmosphere. The discovery of the true nature of these “first generation” F-gases led to the 1987 Montreal Protocol, a multinational treaty which regulated production of CFCs and certain other ozone depleting substances (ODS).
The impending phase-out of CFCs opened the way for substitutes. HCFCs (short for hydrochlorofluorocarbons) picked up the slack, boasting less ozone depletion potential (ODP) by dispensing less reactive chlorine to the stratosphere. They also left less of an imprint on the climate than their predecessors in the form of shorter residence times and reduced global warming potential (GWP).
Yet with ozone being the precious atmospheric resource that it is, any destruction of this protective layer is too much. Besides, HCFCs were only ever intended as a stopgap during the transition to an ozone-safe alternative. After CFC production was completely phased out in 1996, regulatory attention thus turned to the second generation of F-gases, the HCFCs. Current international legislation prohibits HCFC production and importing after 2020 in developed countries and 2030 in developing countries.
The industrialized world then made the switch to third generation HFCs in the 1990s. While still synthetic, HFCs are void of all chlorine and bromine with which to react with ozone in the upper atmosphere. But ozone-friendly does not mean climate-friendly. While they may not coat the atmosphere with ozone depleting compounds, their radiative impact is still enormous. The worst offenders possess atmospheric lifetimes and GWP that exceed some of the early CFC blends.
With the EPA’s latest action, HFCs will now be phased out as well. Taking HFCs out of the air in concert with a transition to more environmentally conscious substitutes could put a dent in US emissions, albeit a slight one. EPA estimates that full implementation of these rules will cut annual greenhouse emissions by up to 64 million metric tons by the year 2025, or the equivalent of the annual energy use of 5.8 million homes. Compared with the 6.8 billion tons the US alone burned in 2014, this cutback seems marginal at best. That said, Climate Central’s coverage suggests that discontinuing the use of just one particularly potent HFC could reap effects on the global scale:
“Paul Blowers, a professor of chemical and environmental engineering at the University of Arizona, said his lab’s research, which has not yet been published, shows that if HFC-134a, which is often used as a refrigerant in small air conditioners, were banned in favor of a climate-friendly alternative, overall global greenhouse gas emissions would fall by 1 percent compared to today’s levels.”
The new rules, which go into effect beginning in 2016, are simply the next evolution of the EPA’s adherence to the Clean Air Act. First passed in 1963 and amended regularly since, this piece of legislation charges the agency with continuous assessment of airborne chemicals and pollutants. Now that HFCs are on the chopping block, perhaps other fluorinated gases will garner regulatory scrutiny.
It turns out that several manmade products, while not as prevalent in the atmosphere, are even more potent than the highest impact HFCs. Sulfur hexafluoride, for example, is commonly referred to as a “super greenhouse gas.” According to the IPCC, SF6 is the most potent greenhouse agent it has evaluated, with a GWP over 22,000 times that of CO2 over a time scale of 100 years, and an atmospheric lifetime of 3,200 years. Meanwhile, certain PFCs, like PFC-14, can last up to 50,000 years, and its greenhouse potential is around 6,500 times that of CO2 over the same 100-year period (see Table 2.14, p. 212).
While not major players in our atmosphere, every little bit helps when we’re dangerously flirting with the oft-warned against 2-degree warming threshold. At a conference in Paris on Tuesday, Thomas Stocker, a professor of climate physics at Bern University, remarked with sobering urgency, “At current emissions, we have a time window of about 20 to 25 years until that budget (of emissions consistent with 2 C) is exhausted.”
Any greenhouse emissions we put into the atmosphere on top of fossil fuel burning accelerates this trajectory. A policy portfolio that addresses the low-hanging fruit while keeping fossilized carbon in the ground and promoting clean energy stands the best chance of warding off future disaster.
Further reading:


Comments