Carbon Dating for the Rest of Us

One of the most extraordinary, not to mention useful, avails of science is its ability to comment with accuracy on the past. Our forays into the terrain of microscopy have provided us a privileged aperture into the reaches of history, a perspective of greater and greater precision, enabling us to demystify and make proper sense of what we see and observe of our natural surroundings. Perhaps most humbling is how scientific inquiry irradiates the imperfect fidelity between our subjective intuitions about reality and its actual nature.
Carbon-14 (or radiocarbon) dating is one such mechanism purposed to improve our understanding of the earth’s past in meaningful ways. As one of the oldest age dating methods in use today, it is unfortunate that C-14 dating is still so misunderstood by the general public. Its primary use is in dating organic material less than 60,000 years old, and it is a capable means toward that end. C-14 dating is neither predicated entirely on assumptions—as many contrarians like to contend—nor is it the single-solution remedy for dating all of the earth’s matter.
The C-14 age dating method was first engineered by Willard Libby while serving as professor of chemistry at the University of Chicago. By recognizing the fixed rate of decay of C-14 material, he became the first to measure its half-life. After a bit of fine-tuning of his formula and measurements, he accurately dated several C-14 samples whose actual dates were known, which subsequently earned him the Nobel Prize for chemistry in 1960.
What is C-14?
Everyone is familiar with the element carbon, the life-sustaining sinew infusing every molecule of our body, the absence of which would result in either a very different form of life, or no life at all. A carbon nucleus contains 6 protons, but the number of neutrons can vary. We call these different variations isotopes. While there are fifteen known carbon isotopes, only three of them are found in nature: carbon-12, carbon-13 and carbon-14. Of those, only one is unstable: C-14. Consisting of 8 neutrons, carbon-14 is a radioisotope, the label given to any atomic substance which decays upon exposure to radiation. Conversely, we refer to C-12 and C-13 as stable (nonradioactive) isotopes since they do not undergo radioactive decay.
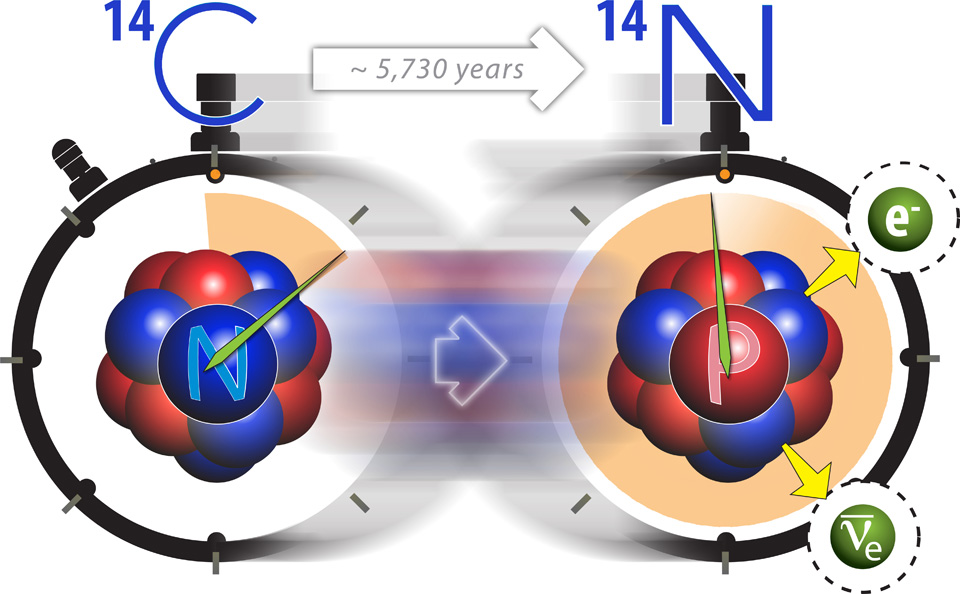
All organic life, whether plant or animal, contains C-14. When exposed to radiation from the sun, C-14 decays into nitrogen-14 (N-14) via beta decay. As long as the organism is alive, regardless of which rung on the food chain it occupies, it will retain a stable supply of C-14 by continually replenishing its stores. Plants absorb C-14 levels via photosynthesis. Humans and animals on the other hand refill their carbon reserves by consuming plants and other animals. This self-renewing cycle of carbon exchange terminates upon the death of the organism.
Equilibrium
Of central importance is not that we simply know that our bodies all contain C-14 but that we know how much is cached within our elemental makeup. We look to the earth’s atmosphere for this answer. Depending on the CO2 concentration of the atmosphere, the ratio of C-14 to C-12 (or other carbon isotopes) will change. Plants (through photosynthesis) and animals (through our robust organic diet) tend to share the same ratio as the atmosphere. That is, the ratio of C-14 (unstable) to C-12 (stable) atoms in the atmosphere persists in most living organisms, both plants and animals, resulting in a carbon equilibrium between the atmosphere and biosphere. For radiometric dating purposes, this is indeed convenient.1
Uniform Decay
The natural equilibrium described above only persists until the animal or plant dies. With no new incoming carbon, the ratio of C-14 to non-radioactive isotopes starts to measurably decline. It was his observation of a predictable decay rate that garnered W. Libby veneration among his peers.
Libby and his team were the first to measure the rate of C-14 decay. Forever canonized as the “Libby half-life,” the original half-life count his team calculated for C-14 was 5568±30 years, which has since been revised to its current value of 5730±40 years. It is important to note that these revisions were the product of using more precise equipment and instrumentation, which brought us closer and closer to the reference value found in nature, and are not reflective of the decay rate fluctuating over time. To date: “No naturally occurring physical or chemical conditions on Earth can appreciably change the decay rate of radioactive isotopes.” Moreover, many lab experiments have taken place in specific attempts to check and re-check decay rates, but these experiments have invariably failed to produce any significant changes. Such is the self-correcting nature of science.
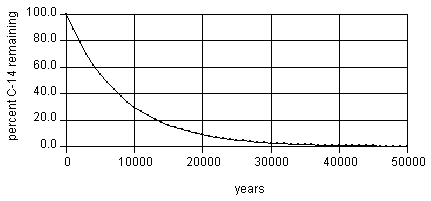
The first-order decay curve of C-14
Carbon-14 decay is measured in terms of exponential decay rates, meaning the rate of decay is dependent upon the number of C-14 atoms present in a sample. If, for instance, the initial value of C-14 is 100 atoms, and we observe a sample with 50 atoms, we can then conclude the sample is approximately 5,730 years old. At 11,460 years, we can expect the sample to have just 25 C-14 atoms, and so on. Generally, the larger the number of C-14 atoms present in a sample, the more reliable the resulting age will be.
In radiometric dating, we determine the initial value by adding the number of daughter atoms present in the sample to the number of parent atoms present in the sample, both of which we can measure. In the case of C-14 dating, we can add the number of N-14 atoms to the atoms of its parent isotope, C-14.
CO2 Variability
Those adequately versed on the present climate crisis are well aware that the concentration of CO2 in the earth’s atmosphere is not a constant variable. Fossil fuel production, deforestation, nuclear weapons testing, cosmic radiation and natural paroxysms like volcanic eruptions all can introduce fluctuations in atmospheric C-14. For example, nuclear tests effected across the globe throughout the 20th century increased the ratio of C-14 temporarily. As it happens, isotopic signatures also represent one of the “smoking guns” for human-caused global warming. Naturally occurring CO2 has a high C-14 ratio, while artificial (i.e., anthropogenic) CO2 from fossil fuel production emits lower C-14 ratios. Analysis of historical atmospheric composition has revealed higher, relatively equable C-14 ratios prior to the Industrial Revolution.
Remember that the ingress and egress of C-14 in organisms is essentially a mirror of atmospheric ratios, so if atmospheric C-14 shifts, thus does animal and plant C-14. Otherwise put, while the C-14 ratio found in living matter tends to stay consistent with the atmospheric ratio, it is the atmospheric ratio itself that is inconsistent over time.
At first glance, this seems to cast doubt on the validity of C-14 dating. However, we in fact have very solid reconstructions of CO2 across geologic time. Ice core analysis, a longstanding technique used in paleoclimatology, provides a window into past CO2 levels, including its isotopic signature. Ice core analyses in recent decades have revealed atmospheric CO2 levels dating back 800 thousand years, denoting relatively steady C-14 ratios prior to fossil fuel proliferation and a downward shift in C-14 following the Industrial Revolution.
Moreover, in 1976 a team of geologists uncovered organic carbon molecules preserved in 22,000 year-old lake sediments. Their discovery corroborated prior and future ice core analyses, demonstrating that the concentration of C-14 in the atmosphere during that interval were largely consistent and did not vary by more than 10 percent.
That means we have very solid empirical evidence for only small deviations in atmospheric C-14 dating back at least 22,000 years, and at most up to C-14’s 58-62,000 year confidence interval.
To compensate for subtle shifts in atmospheric C-14, a correction factor is applied to the dating formula. Keep in mind that these correction factors are still only estimates, though field scientists looking to verify the age can look to external lines of evidence. For instance, in places where a lot of dating has been done, there is usually a fair expectation of what the age of a nearby fossilized specimen will be from the other material in the site. Geochemists regularly employ a variety of other dating methods to corroborate or falsify the results of a C-14-derived age. These include:
- Dendrochronology (“tree-ring dating”)
- Thermoluminesence dating
- Obsidian hydration dating
- Fission track dating
- Other radioisotope dating methods (K-Ar, U-Pb)
These serve as cross-checks for verifying the age of older materials when initial results seem dubious. To be sure, historical isotope ratios represent the greatest uncertainty involved with C-14 age dating, but, again, atmospheric C-14 levels have been shown to not vary appreciably within the extent of C-14’s confidence interval and, secondly, it is standard practice with radiometric dating to err on the side of conservatism wherever estimates are involved.
Misconceptions
Many people misconstrue C-14 dating as having “proved” the earth is not billions of years old or, upon hearing of its limited backward extrapolation of 62,000 years, assume this must be the maximum age of the dinosaurs. Both conclusions miss the mark for two reasons. First, C-14 dating is useful only for organic matter. The currently accepted age of the earth—4.54 billion years—is instead based upon the dating of inorganic material using entirely different radiometric methods. Secondly, carbon dating is incapable of providing reliable ages greater than about 62,000 years, obviously invalidating any applicability to dinosaur fossils. For ages outside this range, we must resort to other dating methods.
Fortunately, other forms of radiometric dating can be used to date fossilized fauna and strata of practically all ages. In fact, some of the isotopes we use in the field have half-lives ranging from 10 years (e.g., tritium) to over 100 billion years (e.g., Samarium-147). If a particular sample contains the necessary isotopic mixtures, even the oldest of strata can be reliably dated.
As the British paleontologist and author Richard Fortey put it when commenting on the 3.5 billion year-old stromatolite fossils discovered in Western Australia:
Sources:
- Explainer: What is an Isotope?
- The Process of Carbon-14 Decay and Dating
- How is Carbon Dating Done?
- Carbon 14 Dating – FAQ
- Dating the Fossil Record
- The Age of the Earth
- Human Caused Global Warming – OSS Foundation
- How Good Are Those Young Earth Arguments?
Feature image credit: Best of Rob. Olduvai gorge, Great rift valley, Tanzania. World-famous dig site for physical anthropologists Louie and Mary Leaky, et al. Many early finds of australopithecenes were made here. That outcrop on the left has been nicknamed ‘the monolith.’
- Many marine animals, such as sea mollusks, as well as plants and animals found near limestone, may have appreciably different ratios of C-14 from the atmosphere due to specific environmental influences and thus C-14 dating is not useful for specimens of this variety.
[↩]


Comments